Resources
DQ IQ OQ PQ
Validation protocols are a method of establishing documented evidence that shows a high degree of assurance that a manufacturing process will consistently yield a product of repeatable high quality.
In line with GMP guidance, we provide Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ) and Perfomance Qualification (PQ) services, to support validation and quality protocols.
What is DQ IQ OQ PQ?
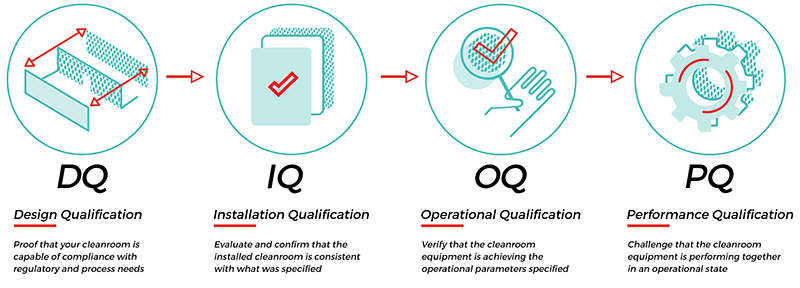
Through the coordination of cleanroom services and equipment, we can get your cleanroom operational faster.