Pharma Cleanrooms for AstraZeneca
AstraZeneca appointed Angstrom Technology to create a cleanroom for the open processing of biological products in its new facility – in the centre of the Cambridge Biomedical Campus (CBC).

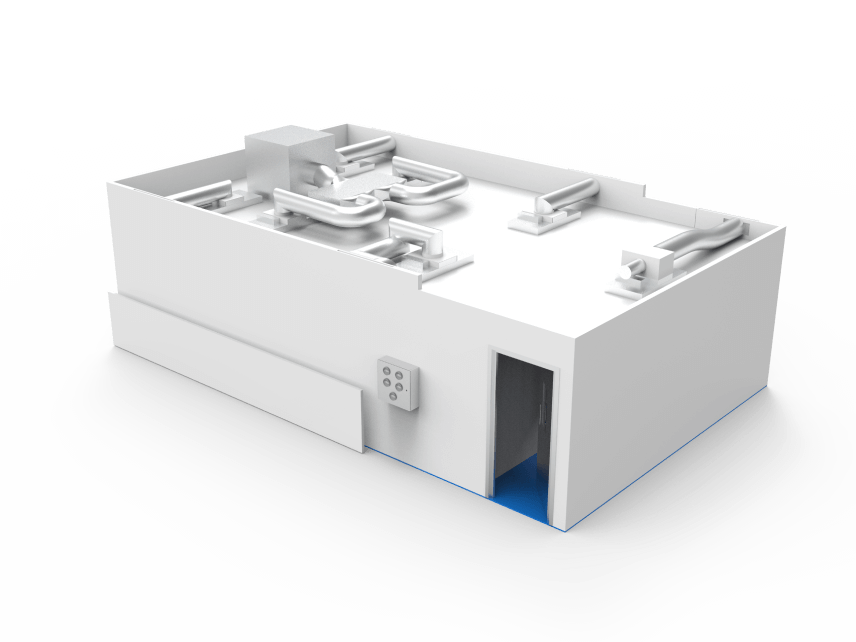
Key facts

12m² cleanroom facility
For the open processing of biological products

Low particulate and low microbial environment
In the Formulation Department of BioProcess Development

ISO Class 6
Dedicated LFU zone operating at ISO Class 5

HEPA filtration
Creating up to 240 air changes per hour
The purpose of the cleanroom is to provide a low particulate and low microbial environment for the open processing of biological products in the Formulation Department of BioProcess Development (BPD).
The cleanroom incorporates a main enclosure operating at ISO Class 6 and a dedicated LFU zone operating at ISO Class 5.

I have worked with Angstrom over the last 2 years with regards to sourcing and procuring clean rooms for a large and complex Pharma project in the Cambridge area.
I have found all the Angstrom team involved in the delivery of this project, to be very knowledgeable, professional and customer focussed throughout the project.

START A PROJECT WITH US
Our design and build specialists have experience working with customers in all kinds of industries on a global scale, achieving great results time and time again. We’d love to work with you as well!
REQUEST A QUOTE