Cell and Gene Therapy Suites
Cell and Gene therapy suites are providing patients in the NHS and private healthcare settings with life changing medicines across the UK and beyond. As the innovative therapies market is maturing, the UK is fast turning into a global leader.


Cell and Gene Therapy
The Cell and Gene Therapy catapult, which has the core purpose of building a world-leading Cell and Gene therapy sector in the UK, reported a 100% increase in phase III clinical trials in the past 2 years alone. With a 60% increase in manufacturing space within the same period, many Advanced Therapy Medicinal Product (ATMP) manufacturers are in growth with expansions planned or in progress.
Innovate UK is strengthening Gene Therapy in the UK by capital investment grants to advance the UK’s ability to produce viral vectors for use as an ATMP or in the development of cell-based ATMPs and to encourage partnerships between public and private organisations, to maximise further investment.
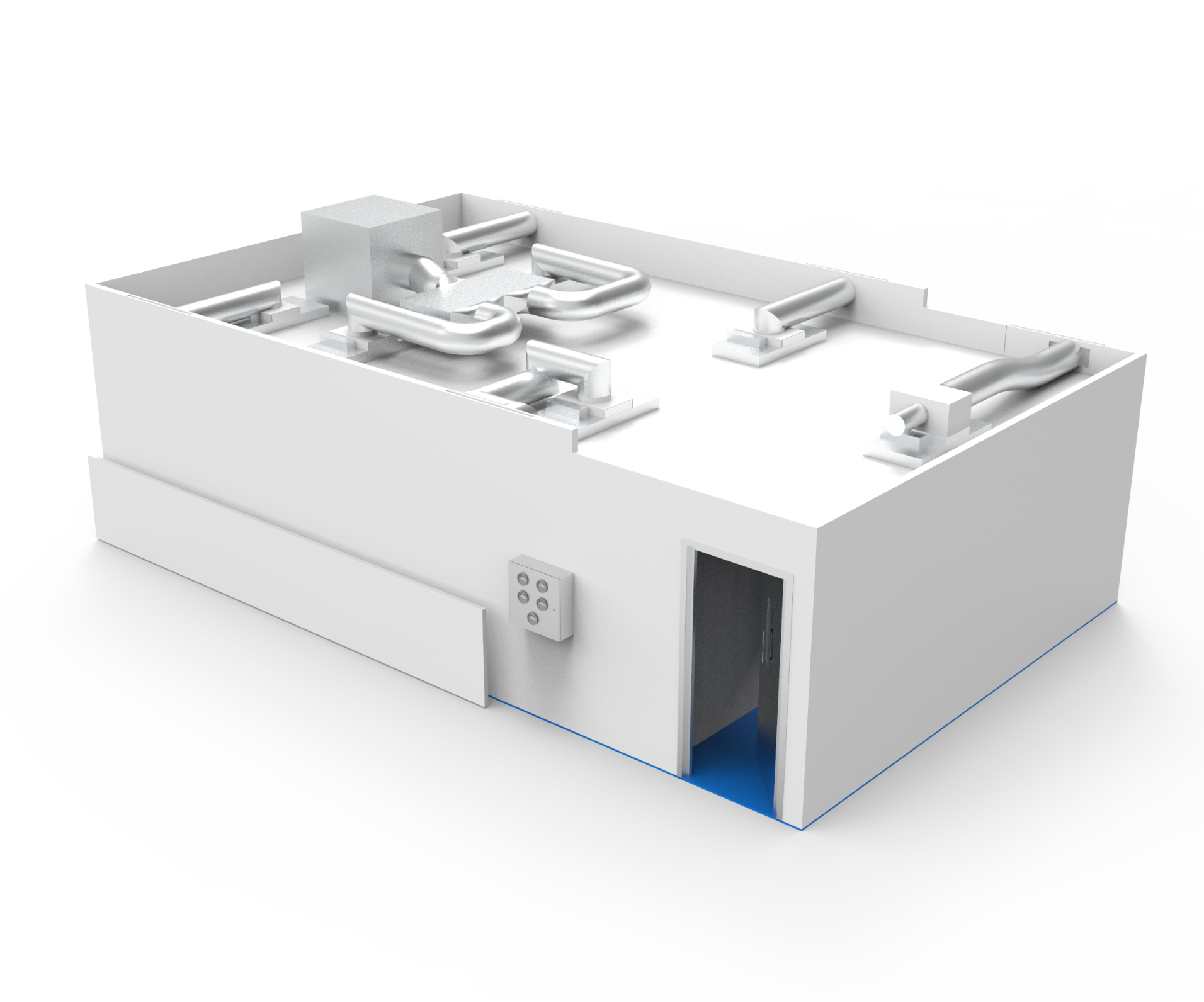
Facility Design and Validation
For new facilities or expansions, it is vital to get all detail of the design correct. For Cell and/or Gene Therapy GMP cleanrooms, the design must integrate process needs with meeting regulatory requirements, to provide an appropriate solution for product, process and people. The integration of utilities, specialist systems and equipment will be critical to the cleanroom design process.
To achieve GMP compliance, MHRA licensing and to meet any other regulatory requirements, the proposed facility will need an initial User Requirement Specification (URS) to fully define the requirements of the facility. A project team should be set up for the creation of the URS, with key stakeholders, including Quality, Production and Facilities Management. This team can review the process requirements, including the flow of people and products through the facility, to assess the optimum layout for regulatory compliance, efficient operation, any segregation requirements and the minimisation of cross contamination.
To meet the requirements of the URS, a detailed and documented design qualification (DQ) programme, including documentation, drawings, technical schedules and specifications, will underwrite the proposed design of the facility. Leading design principles and understanding of the relevant regulatory requirements will keep cleanroom entry, exit and the flow of personnel and materials separated. Sufficient space will be allowed in the layout for incorporating transfer hatches, material airlocks and multiple garment changes.
Functional & mechanical system design of Cell and Gene therapy manufacturing suites will need to be qualified to systematically demonstrate and document that facilities, systems and equipment, perform as intended through Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ) processes.
Technical solution
With many organisations in growth, being creative with space within and external to the facility will maximise available and potential future footprint and retain sufficient space for any future expansion, enabling organisations to scale up from ATMP early phase clinical trials to commercial manufacturing.
In cleanroom design, this can be achieved through implementing more modern methods of air handling. Using a decentralised air handling approach can achieve the same performance as an air handling unit. Benefits range from reduced constructional cost and complexity, to an increase in usable footprint, as a central plant room is not required.
Angstrom Technology was the Principal Contractor on a new Cell and Gene therapy manufacturing facility for Roslin CT.

READY TO START A NEW PROJECT?
Our design and build specialists have experience working with customers in all kinds of industries on a global scale, achieving great results time and time again. We’d love to work with you as well!
REQUEST A QUOTE








